HOME > Reserch
Reserch
Degradable polymersDegradable polymers with low-temperature formability |
Membrane separationsCO2 separation membranes |
Reserch topics
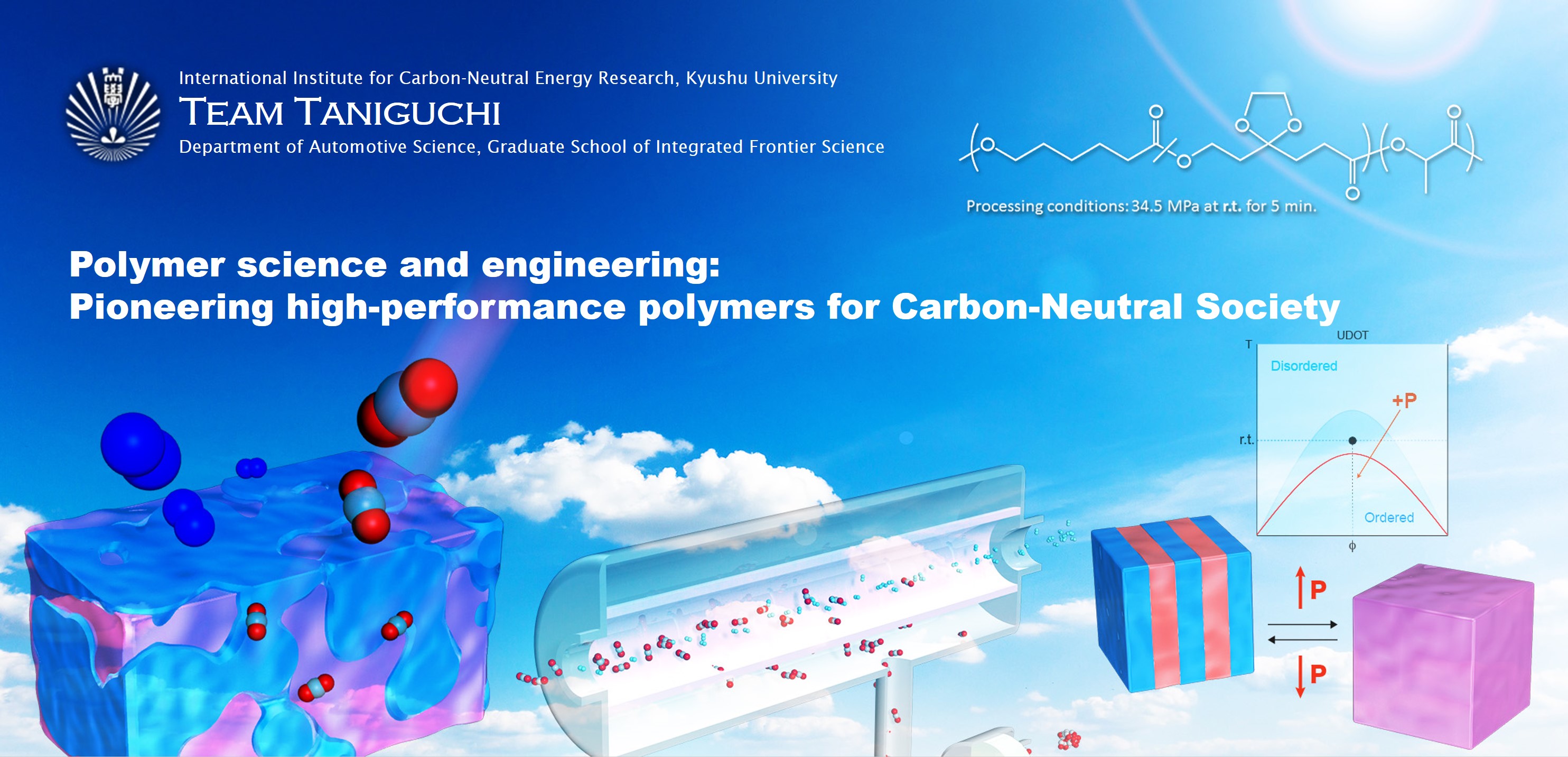
Degradable polymers with low-temperature formability
Multi-component polymers show pressure-induced phase transition at a constant temperature, Baroplastics. Especially, effect of pressure applied on two-component systems, such as block copolymers, has been intensively studied. We have figured out that degradable polymers from polycaprolactones and polylactides exhibit pressure-induced phase miscibility and can be processable at ambient temperature under pressure. The degradable block copolymers have a separated structure (solid state) upon microphase separation at ambient conditions, which is going to a miscible phase (melt state) under pressure. The degradable polymers with low-temperature formability are environmentally benign and drastically reduce required energy in processing, leading to decrease CO2 emission. We also found those polymers prepared from CO2 and other renewable resources, which would hold potential to be “game-changing polymeric materials”.

I. Taniguchi, et al. Macromolecules, 2012
Functionalization of degradable polymers
Polyesters are obtained by ring-opening polymerization of lactones (cyclic esters) and lactides (cyclic dimers of lactic acid), and the properties can be determined by those chemical structures. For chemical modifications, it has been unenviable to introduce functional groups onto the polymer backbone. We have succeeded functionalization of polyesters by grafting functional groups through a chemoselective reaction between aminooxy group and ketone. This chemoselective approach can broaden opportunity of polyesters for use.

- Highly reactive partners tolerate the presence of diverse functional groups.
- Reaction proceeds rapidly under mild reaction condition.
- Two partners react covalently to form stable oxyme linkage.
I. Taniguchi, et al. Macromolecules, 2005
Degradation mechanism of polymers
One of the biggest challenges of degradable polymers is how to control the degradability, which can be characterized by chemical structures, crystallinity, molecular weight, and composition if copolymers. Elucidation of the detail degradation mechanism would provide useful information to design and synthesis of polymeric materials with timed degradation. Biodegradation of hardly degradable polymers, such as PET, is also an important research topic.

S. Yoshida, et al. Science, 2016
CO2 separation membranes
Mitigation of CO2 emission is an urgent issue to suppress the Global warming and Climate change, and various CO2 capture technologies have been examined at a CO2 mass emission source, such as thermal power stations. Membrane separation is an energy saving process and highly expected as a next generation CO2 capture technology. In this laboratory, membrane materials have been prepared for CO2 capture at the various emission sources. Especially, we have developed promising polymeric membranes for CO2 separation over H2. Those polymeric membranes would be applicable not only for pre-combustion CO2 capture at an integrated gasification combined cycle plant but also for carbon-free H2 production at current steam reforming process of light hydrocarbons.

I. Taniguchi, et al. J. Mater. Chem. A, 2013
CO2 capture by electrodialysis
Liquid amine scrubbing, current CO2 capture technology, has been studied for post-combustion CO2 capture at demonstration scale. However, large energy consumption in CO2 recovery limits the implementation. We proposed an alternative CO2 capture method by electrodialysis. CO2 separated in an alkaline solution is condensed by electrodialysis, and the CO2 is then regenerated under vacuum. The required energy for CO2 capture is much lower than that of the current method. A key for further energy reduction is to develop ion-exchange membranes with lower membrane resistances.

I. Taniguchi, et al. J. Chem. Lett., 2014
