| Introduction |
This laboratory conducts researches on “Electrochemistry relevant to Environment and Energy.” Ion-conducting solids, proton-conducting materials in particular, are dealt for practical application as well as development of their functions. The purpose is to contribute to make or community low energy and low pollution, in a way to change the energy source from fossil to renewable energies.
| Hydrogen-Energy Devices Based on Proton-Conducting Metal Oxides |
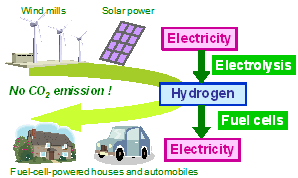
Fig. 1 Hydrogen energy system based on electrolysis and fuel cells.
Solid conducting hydrogen ion (proton) is useful for hydrogen-energy-related devices. Fuel cells, water/steam electrolysis for the production of hydrogen, hydrogen separation, hydrogen sensing and so on are the subjects to study as useful devices for the realization of hydrogen energy community (Fig. 1).
Principle of steam electrolysis using proton-conducting metal oxide electrolyte is shown in Fig. 2. External application of a direct current leads to oxidation of water to generate oxygen gas and proton at the anode (left compartment) and hydrogen gas is generated at the cathode as a result of the reduction of proton at the cathode (right compartment). Energy efficiency should be as high as possible by suppressing the electrode overpotentials and Ohmic loss (electrolyte resistance). Energy efficiency exceeding 90% (HHV) has been attained at 600°C so far. Solid oxide fuel cell (SOFC) using proton conducting oxides is another subject and 600°C operation has been demonstrated (Fig. 3). Electrochemical hydrogen pumping and mixed-conducting hydrogen permeable ceramic membrane for selective hydrogen separation are studied.
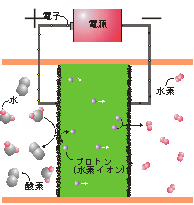
Fig. 2 Principle of steam electrolysis
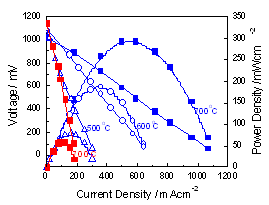
Fig. 3 Fuel cell using proton-conducting oxide.
| Exploration and development of new ion-conducting solids |
High conductivity is the most important property of solid electrolyte materials necessary for the development of high performance electrochemical devices; chemical stability and mechanical strength are needed for practical materials as well. Iwahara discovered a significant proton conduction in perovskite-type metal oxides and reported in 1981. Starting from this material of Japan origination, trials are being made for the new material development.
Ion conduction in solid is entirely dealt with in terms of “bulk conduction” and material designing is based on the concept of bulk defects. That is, ionic species mobile in crystalline materials are always point defects (vacancy and interstitialcy). In recent years, specific ionic properties taking place at interfaces are drawing increasing attention as “nanoionics.” We discovered a notable nanoionics effect in a proton-conducting oxide dispersed with platinum nanoparticles: Conductivity of the material increase and decrease in response to the disappearance and appearance of the platinum particles in orders of magnitude (Fig. 4).
We are also trying to facilitate proton conduction on the surface of metal oxide nanoparticles. Recently developed surface-proton-conducting titania nanoparticles is an example (Fig. 5).
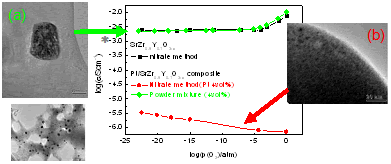
Fig. 4 Nanoionics phenomenon: Conductivity change on the precipitation of platinum nanoparticles in proton-conducting perovskite.
Fig. 5 surface-proton-conducting titania nanoparticles
| Investigation of new phenomena |
Practically useful material may waiting for us somewhere unpredictable merely from the extension of existing materials and theories. For example, we have fund so far a remarkable nanoionics phenomenon to suggest a possible new direction for the development of ion conduction in solids. We will focus on studies looking deep insight of solid state ionics to establish theory and to develop better materials.